Video: Olestra(Olean):Side Effects, Controversies & Researches. How can Cooking Oil be Zero Calorie? How Can Potato Chips Fat Free?
(If subtitles don’t appear or subtitles are not in your language, go to the youtube page and use CC subtitles.)
In addition, if you want to follow more PAAA, welcome to subscribe!
Almost everything nowadays has a diet version, such as diet coke, diet pasta, and diet pizza, which promises a similar flavor to the real thing without the drawbacks, mainly calories.
Sugar substitutes, which can be found on the label of a number of items, are one way for manufacturers to make their products “diet-friendly.” But why isn’t there a fat substitute? Wait, there is indeed one called Olestra!
But here’s the question: If zero-calories cooking oil exists, why it’s not as popular as sugar substitutes? And why was it never sold in a supermarket? This article will explain what Olestra is, a fat substitute developed by P&G. We’ll go through what it is, why it’s not as popular as a sugar substitute, and the controversy surrounding it.
If you’re interested in learning more about the complex relationship between the food industry and nutrition policies, I highly recommend reading Food Politics: How the Food Industry Influences Nutrition and Health (Volume 3) (California Studies in Food and Culture). This book provides valuable insights into the power dynamics and decision-making processes behind food production and regulation, which can help shed light on the controversial history of products like Olestra.
*Please note that I may earn a commission from qualifying purchases made through this affiliate link at no additional cost to you.
What is Olestra(Olean) Zero Calories Fat?
Olestra (also known as Olean) is a fat substitute that is calorie-free. It’s been used to lower or eliminate fat content and calories in foods like potato chips that would otherwise be high in fat.
But how can a cooking oil be fat-free and even calories free you ask? Well, here’s why:
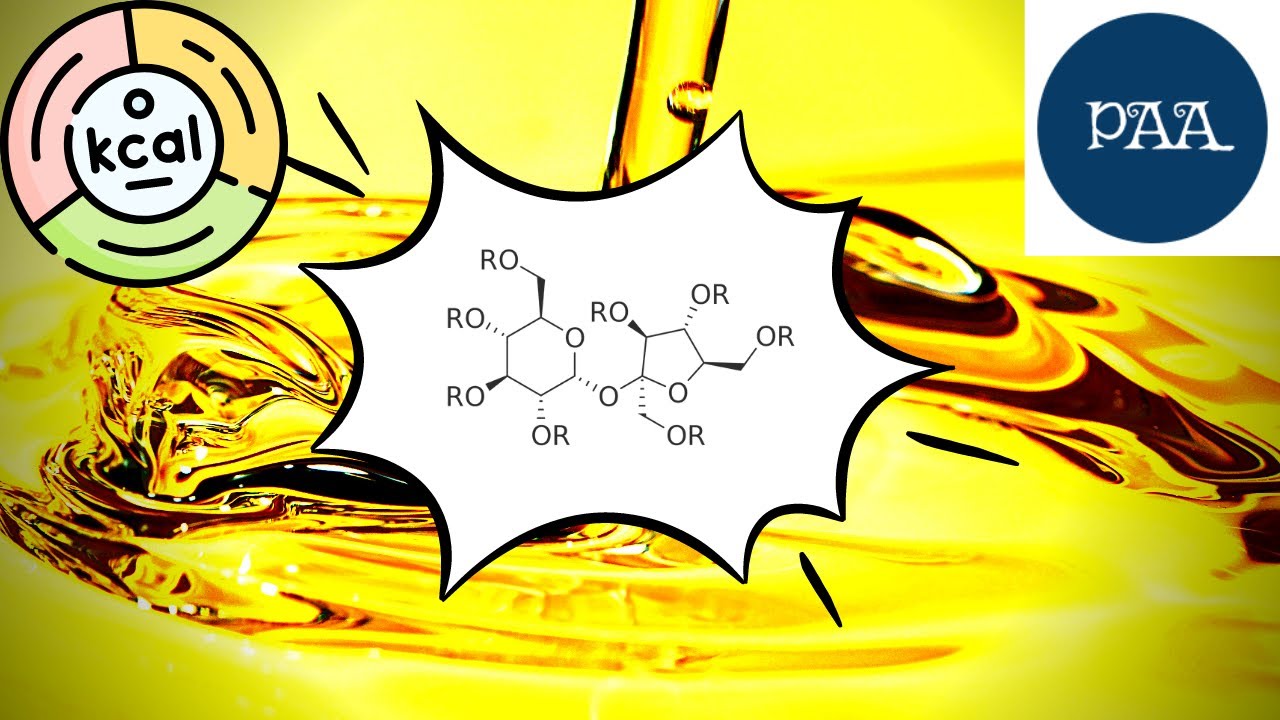
A typical fat consists of two parts: a backbone of glycerol, a type of alcohol, linked to three long-tailed hydrocarbons called fatty acids, which vary in type depending on the fat. Lipases, a type of enzyme found in the intestines, have evolved over millennia to split two fatty acids, leaving truncated molecules small enough to pass through the intestinal wall.
In olestra, however, the glycerol has been replaced by a sucrose molecule, a sugar, which has enough free arms to attach to eight fatty acids. Lipases cannot clamp onto such a large particle, so olestra passes, unscathed, through the intestines. As a result, Olestra tastes like fat but contains, in effect, no usable calories.
In 1996, the Food and Drug Administration (FDA) approved olestra for use in packed ready-to-eat snacks in the United States, ruling that such use “meets the safety standard for food additives, reasonable certainty of no harm.” This conclusion, however, is extremely controversial, as we will discuss later in this article.
Commercialization and Controversies of Olestra
Olestra was discovered by accident in 1968 by Procter & Gamble (P&G) researchers F. Mattson and R. Volpenhein while seeking fats that were easier to digest for premature infants. P&G then talked with the Food and Drug Administration (FDA) in 1971 to discuss the types of testing required to introduce olestra as a food ingredient.
As a function of olestra substituting natural dietary fats, P&G found a decrease in blood cholesterol levels during subsequent tests. Following this potentially profitable opportunity, Procter & Gamble petitioned the FDA to get the substance approved as a cholesterol-lowering medicine in 1975. However, the following human trials revealed that olestra could not cut cholesterol levels sufficiently to warrant this use, the application was then dropped in 1988.
Around the same time, the company applied to the FDA to get olestra approved as a food additive as a substitute for shortening, cooking oil, and snacks. According to the FDA, the company modified the application two years later to limit olestra’s usage to only snacks.
In 1984, the FDA allowed Kellogg to publicly claim that their high-fiber breakfast cereals helped to reduce cancer incidence, motivating P&G to immediately start a three-year test series in a hope that the FDA will also allow health claims for Olestra given some sort of health benefits is found. Once these tests were finished, P&G sought approval as a food additive for up to 35 percent fat substitution in-home cooking and 75 percent in commercial use.
One of the FDA’s major concerns regarding olestra was that it would encourage people to eat more “top of the pyramid” foods (AKA Fats, Oils & Sweet) since they could then be perceived to be healthier. This could lead to overconsumption by people who believe that adding olestra will eliminate undesirable health consequences.
Given this probability, approving it as an ingredient would have resulted in people consuming food containing a rather high dose of an additive with unknown long-term health impacts. As a result, as well as side effects like diarrhea and concerns about fat-soluble vitamin loss, the FDA was hesitant to approve the product for less restrictive use. In August 1990, P&G focused on “savory snacks,” such as potato chips and tortilla chips.
At the time, the original patents were set to expire in 1995. In December 1993, P&G successfully pushed for and received an extension. This extension was valid until January 25, 1996. Due to P&G’s pressure on FDA, clearance was granted on January 24, one day before the patent expired, extending the patent for another two years automatically.
FDA concluded at the time of the 1996 ruling that
“to avoid being misbranded… olestra-containing foods would need to bear a label statement to inform consumers about possible effects of olestra on the gastrointestinal system. The label statement also would clarify that the added vitamins were present to compensate for any nutritional effects of olestra, rather than to provide enhanced nutritional value”
The FDA later withdrew the label, claiming that it was misleading since FDA agreed with P&G that the “label statement could be misleading and cause consumers of olestra to attribute serious problems to olestra when this was unlikely to be the case”.
What are the Side Effects of Olestra (Olean)?
The drawback of Olestra is that it may result in loose bowels, flatulence, and cramping in the intestines. Additionally, it may prevent the body from absorbing carotenoids, which are believed to lower the risk of developing cancer. Olestra also prevents the body from absorbing vitamins A, D, E, and K.
According to an article published in the Journal of the American Dietetic Association in 1998, FDA Food Advisory Committee, and public discussion made a controversial decision on January 25, 1996, to allow olestra to be used in savory snacks (eg, salty snacks such as potato chips, corn chips). Because olestra may interfere with the absorption of vitamins A, D, E, or K when taken with meals containing these vitamins, the FDA is requiring that the fat-soluble vitamins lost be added back to olestra-containing products in the manner specified below:
170 IU vitamin A per gram olestra, 12 IU vitamin D per gram olestra, 2.8 IU vitamin E per gram olestra, and 8 micrograms vitamin K per gram olestra.
Center for Science in the Public Interest(CSPI), a Washington, D.C.-based non-profit watchdog and consumer advocacy group that advocates for safer and healthier foods gives Olestra a rating of “avoid” on their website, given that Olestra’s side effects, including gas, cramping in the abdomen, diarrhea, and loose stools can sometimes be serious.
CSPI argue that while manufacturers can sell greasy-tasting low-fat snacks with Olestra, baked foods are safer alternative for calorie-saving, and because olestra includes significant levels of indigestible fat, products made with olestra shouldn’t be marketed as “fat free.”
Does olestra mimic a true fat?
The answer to this question is extracted from an article titled “The Chemistry of . . . Fat Substitutes” published by Discover Magazine, which is an American science magazine launched in October 1980 by Time Inc. and has been owned by Kalmbach Publishing since 2010.
According to this article, a typical fat consists of two parts: a backbone of glycerol, a type of alcohol, linked to three long-tailed hydrocarbons called fatty acids, which vary in type depending on the fat.
Lipases, a type of enzyme found in the intestines, have evolved over millennia to split two fatty acids, leaving truncated molecules small enough to pass through the intestinal wall. In olestra, however, the glycerol has been replaced by a molecule of sucrose, a sugar, which has enough free arms to attach to eight fatty acids. Lipases cannot clamp onto such a large particle, so olestra passes, unscathed, through the intestines.
Olestra tastes like fat but contains, in effect, no usable calories, and two studies— both funded by Procter & Gamble— suggest that consuming it can be helpful to those with high cholesterol or heart problems. One of the studies indicates that people who eat olestra can reduce their cholesterol levels by more than 10 percent; the other study found a more than 10 percent increase in blood flow to the heart among people who had just eaten an olestra-filled banana muffin.
Here I want you to notice two things, first, the article mentioned this research was published in 2001, so they are old studies that might be outdated.
Second, these studies were founded by P&G which is the exact company that was trying to commercialize olestra, so I’ll take these findings with a grain of salt.
So let’s talk about its controversy in our next PAA:
Can we digest olestra?
Google’s auto-generated answer is linked to an article titled “Olestra Controversy” published by The Canadian Encyclopedia which is the national encyclopedia of Canada, published online by the Toronto-based historical organization Historica Canada.
And another article titled “Olestra Eaters, Beware” published by WebMD
According to these two articles, olestra might soak up and eliminate essential vitamins and nutrients. It may also cause flatulence and diarrhea because, well, if fat cannot be absorbed by the body it has to go somewhere eventually.
But in 1996, despite strong objections by nutritionists and consumer advocates, the United States Food and Drug Administration approved olestra for use in salty snacks and crackers – provided they carry a label warning consumers of possible digestive upsets.
Reporting in the Feb 15, 2000 issue of the Annals of Internal Medicine, a research team led by Ranga Balasekaran, MD, also found that people who regularly consume olestra-containing potato chips may test false-positive for steatorrhea because they are likely to have high amounts of fat in their feces due to the unique chemical composition of olestra, leading to expensive and unnecessary tests.
So, here’s the question:
Is Olestra Still Used in Food?
Google’s auto-generated answer is linked to an article titled “6 foods that are legal in the US but banned in other countries” published by Insider in 2017.
According to this article, since Olestra has been linked to gastrointestinal disease in children, and terrible diarrhea in adults and has also been found to increase appetite, completely negating its potential fat-free benefits, it’s banned in Canada and European countries.But you might find Olestra,sometimes referred to by its brand name Olean, in American foods. I just never saw one personally, I wonder where I could find one.
Is Olestra Fat-Free?
Center for Science in the Public Interest(CSPI), a Washington, D.C.-based non-profit watchdog and consumer advocacy group that advocates for safer and healthier foods argue that while manufacturers can sell greasy-tasting low-fat snacks with Olestra, baked foods are safer alternative for calorie-saving, and because olestra includes significant levels of indigestible fat, products made with olestra shouldn’t be marketed as “fat-free.”
What are Some Books related to Food Safety and Food Politic?
By the way, since you are here chances are that you might be interested in some Food Safety and Food Politic related information, so here’s for some further reading recommended. Of course, those are affiliate links but those are the books I genuinely think are interesting :
- Food Politics: How the Food Industry Influences Nutrition by Marion Nestle (2002) is a comprehensive overview of the food industry and its impact on our health. The book discusses the role of food marketing, government regulations, and corporate influence on the food we eat.
- Salt Sugar Fat: How the Food Giants Hooked Us by Michael Moss (2013) is a well-researched book that exposes the tactics used by the food industry to get us hooked on unhealthy foods. The book discusses the use of sugar, salt, and fat in processed foods, as well as the marketing strategies used to make these foods irresistible.
- The Omnivore’s Dilemma: A Natural History of Four Meals by Michael Pollan (2006) is a fascinating exploration of the food we eat, from the farm to the table. The book examines the different ways that food is produced and processed, and the implications of our food choices for our health and the environment.
- Fast Food Nation: The Dark Side of the All-American Meal by Eric Schlosser (2001) is a critical look at the fast food industry. The book exposes the unhealthy ingredients used in fast food, the low wages paid to workers, and the environmental impact of the industry.
- The Dorito Effect: The Surprising New Science of Food Cravings by Mark Schatzker (2015) is a look at the science behind food cravings. The book discusses the role of taste, smell, and hormones in our food choices, as well as the marketing strategies used to make us crave unhealthy foods.
- The End of Overeating: Taking Control of the Insatiable American Appetite by David Kessler (2009) is a book about obesity and how to prevent it. The book discusses the factors that contribute to obesity, such as our environment, our genes, and our food choices.
Also learn about Canola Oil :Video: Why Canola oil is NOT Banned in Europe?
Further Reading and References:
- Prince, D. M., & Welschenbach, M. A. (1998). Olestra: a new food additive. Journal of the American Dietetic Association, 98(5), 565–569. https://doi.org/10.1016/s0002-8223(98)00126-6